Rocznik Ochrona Środowiska 2025, vol. 27, pp. 1-12
![]() Syahril Nedi
Syahril Nedi ![]() ,
, ![]() Irwan Effendi
Irwan Effendi ![]() ,
, ![]() Afrizal Tanjung
Afrizal Tanjung ![]() ,
, ![]() Ronal Kurniawan
Ronal Kurniawan ![]() ,
, ![]() Elizal Elizal
Elizal Elizal ![]()
| University of Riau, Indonesia | |||
| |
|||
| |
https://doi.org/10.54740/ros.2025.001 | ||
| Received: August 2024 | Accepted: December 2024 | Published: January 2025 |
The research aimed to measure the effectiveness of Azolla microphylla in reducing petroleum hydrocarbon in brackish water. A completely randomized design was used with two factors: pollutant concentration (factor A) and A. microphylla addition (factor B). A consisted of A1 (10 ppm), A2 (20 ppm), A3 (30 ppm), A41 (40 ppm), and A5 (50 ppm). B consisted of B1 (50 g) and B0 (0 g of A. mycrophilla). The experimental units contained 10 L of water planted with fern (50 g) and control (B0). Analysis of variance shows that A.microphylla has a significant effect on the reduction of petroleum hydrocarbons in brackish water. The plant reduced levels of this pollutant by up to 36%. Without A. microphylla plants, the pollutant was decreased to 14-22% only. The pollutant reduced the leaf chlorophyll of the fern to 39.5%. The higher the pollutant level, the lower the chlorophyll content.
| |
bioremediation, chlorophyll, dissolved oxygen, A. microphylla, oil pollution, pollution reduction
1. Introduction
Petroleum hydrocarbon pollution is a significant environmental concern caused by releasing petroleum products into the environment, often due to spills, leaks, or improper disposal. Control of petroleum hydrocarbon pollution in waters has been carried out mechanically, chemically, and biologically. Effective treatment methods vary depending on the extent of contamination, the type of hydrocarbons involved, and the environmental context. Some common treatment approaches include physical, chemical, thermal, and biological methods. Physical methods may include 1) containment and recovery using oil booms and skimmers are used to contain and recover free-floating hydrocarbons from water surfaces. 2) Excavation of contaminated soil can be excavated and removed for treatment or disposal. 3) Soil washing involves using water and/or chemical agents to wash out contaminants from soil. 4) Soil vapor extraction (SVE) techniques can evaporate volatile organic compounds from contaminated soils.
Chemical methods may involve oxidation since some chemical oxidants (like hydrogen peroxide or potassium permanganate) can break down hydrocarbons into less harmful substances. The use of surfactants can enhance the solubility of hydrocarbons, making them easier to extract from soils and sediments. Some chemical stabilization can immobilize pollutants and reduce their bioavailability in contaminated media. Biological methods include bioremediation and phytoremediation. This natural process uses microorganisms to degrade petroleum hydrocarbons. It can occur in situ (at the contamination site) or ex situ (after removing contaminated material for treatment). While phytoremediation involves some plants absorbing and degrading petroleum hydrocarbons through their root systems, making this a sustainable option for remediation.
Dehnavi & Ebrahimipour (2022) stated that bioremediation technology is an efficient, cost-effective and environmentally friendly method. Bioremediation is the safest for the environment (Nedi et al. 2010). Phytoremediation is easy to apply by utilizing the ability of aquatic plants to control hydrocarbon petroleum pollutants. (Wang et al. 2017). The selection of plant types that can reduce hydrocarbon pollutants is important for controlling water quality (Galal et al. 2018).
The Mediterranean seaweed Caulerpa prolifera, in conjunction with epiphytic bacteria, exhibits resistance and diesel degradation capabilities, especially in moderately polluted environments, by changing the bacterial community structure to enhance hydrocarbon breakdown (Caronni et al. 2023, Effendi et al. 2020). Additionally, water hyacinth (Eichhornia crassipes) has been shown to reduce TPH (total petroleum hydrocarbons) by 79%, significantly improving water quality by increasing dissolved oxygen levels (Nedi et al. 2023). Similarly, Neptunia oleracea, has shown the ability to survive in liquid petroleum waste and reduce ammonia and sulfide levels, indicating its potential as a phytoremediation agent for liquid petroleum waste (Hardestyariki & Fitria 2023).
Earlier studies suggested that Azolla filiculoides can tolerate crude oil concentrations of up to 0.2%, with biodegradation rates of total aliphatic and aromatic hydrocarbons reaching up to 94% and 81%, respectively, at these concentrations (Kösesakal et al. 2016, Kumari et al. 2019). Further research showed that A. filiculoides can effectively degrade polycyclic aromatic hydrocarbons (PAHs) in freshwater contaminated with crude oil. The bioremediation potential of A. filiculoides is concentration-dependent, with the most effective removal observed at crude oil concentrations ranging from 0.05% to 0.2% (Kösesakal 2018).
Among the various species, A. microphylla has shown particular promise due to its rapid growth, nitrogen fixation ability, and pollutant reduction effectiveness, making it an ideal candidate for this study. One of the advantages of this plant is Azolla's fast growth rate, with a doubling time of 2-5 days under optimal conditions. One of the advantages of this plant is Azolla's fast growth rate, with a doubling time of 2-5 days under optimal conditions. Increasing its utility in large-scale applications makes it a viable option for sustainable bioremediation efforts (Golzary et al. 2019). This fern absorbs large amounts of atmospheric CO2, which can be converted into biomass for various applications, including biofuel production (Hamdan & Houri 2022). In addition, Azolla's ability to grow in wastewater and reduce contaminants such as NH4–N, NO3–N, PO4–P, and selenium further underscores its utility in environmental remediation (Miranda et al. 2016).
Azolla filiculoides and Landoltia punctata have been used to complement each other in wastewater treatment, achieving phosphate reductions of up to 93% and demonstrating their potential in treating various pollutants, including hydrocarbons (Miranda et al. 2020). Other researchers report this bioremediation ability is further supported by the ability of A. pinnata to degrade TPH up to 92% in petroleum-polluted freshwater, significantly outperforming unplanted controls (Mostafa et al. 2021). Among the various species, A. microphylla has shown particular promise due to its rapid growth, nitrogen fixation ability, and pollutant reduction effectiveness, making it an ideal candidate for this study. This study, therefore, aims to evaluate the effectiveness of A. microphylla in reducing petroleum hydrocarbon pollutants in brackish water environments, building on its demonstrated potential in bioremediation applications.
2. Methodology
2.1. Time and place
This experimental research was conducted from November 2023 to January 2024. Brackish water was collected from the Port of Dumai, Indonesia (Figure 1). A. microphylla was taken from the Marine Biology Laboratory, Riau University collection. Experiments and analyses of samples were carried out at PT's field laboratory. Lagio, Pekanbaru, Indonesia.
Fig. 1. Research location in Dumai City, Indonesia
2.2. Research methodology
This study used a completely randomized design (CRD) with two factors, namely factor A (concentration of petroleum hydrocarbon) and factor B (addition of A. mycrophilla). Factor A consists of five levels, namely A1 (10 ppm), A2 (20 ppm), A3 (30 ppm), A41 (40 ppm), and A5 (50 ppm). Factor B consists of 2 levels, namely B1 (addition of 50 g of A. microphylla) and B0 (control or without A. microphylla) (Sekhar et al. 2019).
2.3. Research procedures
This research consists of 3 stages, namely a) preparation stage, b) preliminary tests, and c) implementation of trials. a) the preparation stage includes providing all 20 L basin equipment, water checker, and portable chlorophyll meter type KWF YLS. Petroleum hydrocarbons are Pertamina DEX (diesel fuel with the best quality and low sulfur content produced by PT Pertamina Persero), seawater (salinity 3%o), and distilled water for dilution. b) preliminary test stage (14 days), namely a preliminary test to determine the range of petroleum hydrocarbon concentrations used in carrying out the test (lower threshold concentration and lower threshold concentration). Preliminary tests were carried out after the acclimatization process was carried out. Acclimatization is the adaptation process (14 days) of A. microphylla plants to the test media environment. c) test implementation stage. The preliminary test results determined a concentration range of 10-50 ppm as the petroleum hydrocarbon concentration. A rectangular container with a volume of 20 L (30 units) was filled with 10 L of 3 ppt salinity water, and A. mycrophilla was placed into and maintained for 14 days (Nedi et al. 2023). This is intended to see the effectiveness of reducing total levels of petroleum hydrocarbons by A. mycrophilla at different concentrations. All treatment units were repeated 3 times, and the position of each unit was placed randomly. The ability of A. mycrophilla to release organic compounds into the rhizosphere, such as amino acids and fatty acids, supports microbial communities that degrade hydrocarbons, thus enhancing the bioremediation process (Kösesakal 2018). This symbiotic relationship between plant and microbes contributes to the observed reductions in hydrocarbon concentrations.
2.4. Measurement of TPH and chlorophyll content
Total petroleum hydrocarbons (TPH) analysis was conducted on days 0 to 7 and 14, using the ASTM 7066-04 method with FTIR (Fourier Transform Infra Red). Chlorophyll levels were also measured for the same period using the KWF YLS-A Portable Chlorophyll Meter, GJS, Germany (Nedi et al. 2023).
2.5. Water quality measurement
Observations of water quality parameters (temperature, pH, dissolved oxygen, and salinity) were carried out every day (9.00 AM) in situ using a Horiba U-52G-10 Multi-Parameter Water Quality Meter water quality checker (Nedi et al. 2023).
2.6. Data analysis
Data from measurements of TPH, chlorophyll content of A. microphylla plant leaves, and water quality parameters were tabulated in tabular form and analyzed statistically through analysis of variance (ANOVA), regression, and correlation at a significance level of 99 and 95%. All findings were then discussed descriptively.
3. Result and Discussion
3.1. Reduction of petroleum hydrocarbons
The results of this study indicate that A. microphylla influences reducing petroleum hydrocarbon levels in brackish water for all concentrations. The initial concentration of each experimental treatment also influenced the percentage reductions. The decrease continued to occur during the experiment. The average content in the 10 ppm treatment A1B1 was lower than A1B0 both on day 7 (6.033 < 9.877) and on day 14 (3.967 < 5.410). The same thing also happened in the treatment with a petroleum hydrocarbon content of 20 ppm, where in experimental unit A2B1, the petroleum hydrocarbon content was lower than A2B0 both on day 7 (11.077 < 17.244) and day 14 (8.047 < 9.299). In the experimental unit with a petroleum hydrocarbon content of 30 ppm, the average A3B1 was lower than A3B0 both on day 7 (15.950 < 24.927) and day 14 (11.077 < 13.878). A similar phenomenon has been observed in treatments with 40 and 50 ppm hydrocarbon petroleum levels, both on day 7 and day 14. More detailed data is presented in Table 1.
Table 1. Reduction of petroleum hydrocarbon concentrations (ppm) by A. microphylla in brackish water
|
No. Treatments |
0 days |
7 days |
14 days |
|
1. A1B1 (PH at 10 ppm with AM) |
10.000 |
6.130 |
4.000 |
|
10.000 |
5.990 |
3.890 |
|
|
10.000 |
5.980 |
4.010 |
|
|
Average |
10.000 |
6.033 |
3.967 |
|
Standard of deviation |
0.000 |
0.084 |
0.067 |
|
2. A1B0 (PH at 10 ppm without AM) |
10.000 |
9.822 |
5.411 |
|
10.000 |
9.986 |
5.387 |
|
|
10.000 |
9.822 |
5.433 |
|
|
Average |
10.000 |
9.877 |
5.410 |
|
Standard of deviation |
0.000 |
0.077 |
0.023 |
|
3. A2B1 (PH at 20 ppm with AM) |
20.000 |
11.232 |
8.142 |
|
20.000 |
11.020 |
8.070 |
|
|
20.000 |
10.980 |
7.930 |
|
|
Average |
20.000 |
11.077 |
8.047 |
|
Standard of deviation |
0.000 |
0.135 |
0.108 |
|
4. A2B0 (PH at 20 ppm without AM) |
20.000 |
17.245 |
8.972 |
|
20.000 |
16.244 |
9.678 |
|
|
20.000 |
18.242 |
9.246 |
|
|
Average |
20.000 |
17.244 |
9.299 |
|
Standard of deviation |
0.000 |
0.999 |
0.356 |
|
5. A3B1 (PH at 30 ppm with AM) |
30.000 |
15.821 |
11.032 |
|
30.000 |
16.050 |
10.976 |
|
|
30.000 |
15.980 |
11.223 |
|
|
Average |
30.000 |
15.950 |
11.077 |
|
Standard of deviation |
0.000 |
0.117 |
0.130 |
|
6. A3B0 (PH at 30 ppm without AM) |
30.000 |
24.654 |
13.432 |
|
30.000 |
25.142 |
14.214 |
|
|
30.000 |
24.986 |
13.987 |
|
|
Average |
30.000 |
24.927 |
13.878 |
|
Standard of deviation |
0.000 |
0.249 |
0.402 |
|
7. A4B1 (PH at 40 ppm with AM) |
40.000 |
18.990 |
15.030 |
|
40.000 |
19.070 |
14.295 |
|
|
40.000 |
18.696 |
15.020 |
|
|
Average |
40.000 |
18.919 |
14.782 |
|
Standard of deviation |
0.000 |
0.197 |
0.421 |
|
No. Treatments |
0 days |
7 days |
14 days |
|
8. A4B0 (PH at 40 ppm without AM) |
40.000 |
32.472 |
17.273 |
|
40.000 |
32.212 |
16.652 |
|
|
40.000 |
34.124 |
17.875 |
|
|
Average |
40.000 |
32.936 |
17.267 |
|
Standard of deviation |
0.000 |
1.037 |
0.612 |
|
9. A5B1 (PH at 50 ppm with AM) |
50.000 |
23.000 |
19.050 |
|
50.000 |
23.050 |
20.030 |
|
|
50.000 |
22.950 |
20.020 |
|
|
Average |
50.000 |
23.000 |
19.700 |
|
Standard of deviation |
0.000 |
0.050 |
0.563 |
|
10. A5B0 (PH at 50 ppm without AM) |
50.000 |
43.250 |
24.820 |
|
50.000 |
42.870 |
26.110 |
|
|
50.000 |
43.240 |
24.210 |
|
|
Average |
50.000 |
43.120 |
25.047 |
|
Standard of deviation |
0.000 |
0.217 |
0.970 |
PH – petroleum hydrocarbon; AM – Azolla microphylla
The decrease in petroleum hydrocarbon levels will be seen even more clearly if the data in Table 1 is converted into percentages for each experimental unit. The highest remaining pollutant content was 99.9% on day 7 of A1B0 treatment, and the lowest was in A4B1 treatment on day 14 (35.7%). The complete analysis data is contained in Table 2.
Table 2. Reduction of petroleum hydrocarbon concentrations (%) by A. microphylla in brackish water
|
No. Treatment |
0 Day |
7 day |
14 day |
|
1. A1B1 (PH at 10 ppm with AM) |
100.000 |
61.300 |
40.000 |
|
100.000 |
59.900 |
38.900 |
|
|
100.000 |
59.800 |
40.100 |
|
|
Average |
100.000 |
60.333 |
39.667 |
|
Standard of deviation |
0.000 |
0.839 |
0.666 |
|
2. A1B0 (PH at 10 ppm without AM) |
100.000 |
98.220 |
54.110 |
|
100.000 |
99.860 |
53.870 |
|
|
100.000 |
98.220 |
54.330 |
|
|
Average |
100.000 |
98.767 |
54.103 |
|
Standard of deviation |
0.000 |
0.947 |
0.230 |
|
3. A2B1 (PH at 20 ppm with AM) |
100.000 |
56.160 |
40.710 |
|
100.000 |
55.100 |
40.350 |
|
|
100.000 |
54.900 |
39.650 |
|
|
Average |
100.000 |
55.387 |
40.237 |
|
Standard of deviation |
0.000 |
0.677 |
0.539 |
|
4. A2B0 (PH at 20 ppm without AM) |
100.000 |
86.225 |
44.860 |
|
100.000 |
81.220 |
48.390 |
|
|
100.000 |
91.210 |
46.230 |
|
|
Average |
100.000 |
86.218 |
46.493 |
|
Standard of deviation |
0.000 |
4.995 |
1.780 |
|
5. A3B1 (PH at 30 ppm with AM) |
100.000 |
52.737 |
36.773 |
|
100.000 |
53.500 |
36.587 |
|
|
100.000 |
53.267 |
37.410 |
|
|
Average |
100.000 |
53.168 |
36.923 |
|
Standard of deviation |
0.000 |
0.391 |
0.432 |
|
6. A3B0 (PH at 30 ppm without AM) |
100.000 |
82.180 |
44.773 |
|
100.000 |
83.807 |
47.380 |
|
|
100.000 |
83.287 |
46.623 |
|
|
Average |
100.000 |
83.091 |
46.259 |
|
Standard of deviation |
0.000 |
0.831 |
1.341 |
|
No. Trearment |
0 Day |
7 day |
14 day |
|
7. A4B1 (PH at 40 ppm with AM) |
100.000 |
47.475 |
37.575 |
|
100.000 |
47.675 |
35.738 |
|
|
100.000 |
46.740 |
37.550 |
|
|
Average |
100.000 |
47.297 |
36.954 |
|
Standard of deviation |
0.000 |
0.492 |
1.054 |
|
8. A4B0 (PH at 40 ppm without AM) |
100.000 |
81.180 |
43.183 |
|
100.000 |
80.530 |
41.630 |
|
|
100.000 |
85.310 |
44.688 |
|
|
Average |
100.000 |
82.340 |
43.167 |
|
Standard of deviation |
0.000 |
2.593 |
1.529 |
|
9. A5B1 (PH at 50 ppm with AM) |
100.000 |
46.000 |
38.100 |
|
100.000 |
46.100 |
40.060 |
|
|
100.000 |
45.900 |
40.040 |
|
|
Average |
100.000 |
46.000 |
39.400 |
|
Standard of deviation |
0.000 |
0.100 |
1.126 |
|
10. A5B0 (PH at 50 ppm without AM) |
100.000 |
86.500 |
49.640 |
|
100.000 |
85.740 |
52.220 |
|
|
100.000 |
86.480 |
48.420 |
|
|
Average |
100.000 |
86.240 |
50.093 |
|
Standard of deviation |
0.000 |
0.433 |
1.940 |
The results of variance analysis show that A. microphylla significantly affects the reduction of petroleum hydrocarbons, where the F value is greater than the F critical (9.323 > 4.413). Time exposure also has a very significant effect, where petroleum hydrocarbon levels decrease with time, and the statistical analysis results show that Fvalue is greater than Fcritc (189.389 > 3.101). Without A. microphylla, media containing petroleum hydrocarbon pollutants also experienced a reduction of 40.237-54.330%. Phytoremediation of petroleum hydrocarbons on day 7 was around 40-54%, and on day 14, there was an increase of 60-63.3%. The optimum reduction of petroleum hydrocarbon pollutants by A. microphylla plants occurred at a concentration of 30 ppt on the 14th day. Figures 2 and 3 illustrate the decreasing trends in hydrocarbon concentrations and chlorophyll content over time, underscoring the effectiveness of A. mycrophylla in pollutant reduction and the associated physiological impact.
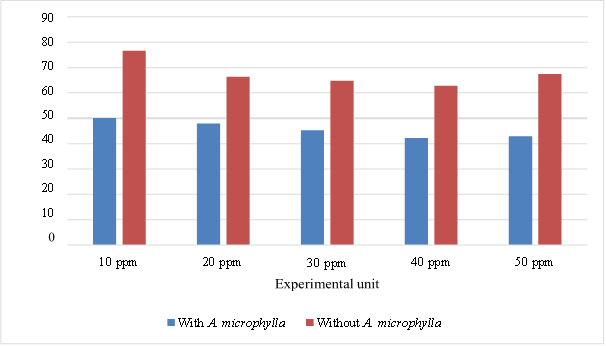
Fig. 2. Reduction of petroleum hydrocarbon concentrations (%) by A. microphylla in brackish water
The decrease in petroleum hydrocarbon concentration is directly related to the length of the test time, which affects the ability of A. microphylla to absorb hydrocarbons. Apart from that, the decrease in petroleum hydrocarbons was significantly different at each concentration, which is thought to be due to the absorption process by plant roots in the rhizosphere area. The ability of A. microphylla to release organic compounds into the rhizosphere, such as amino acids and fatty acids, supports microbial communities that degrade hydrocarbons, thus enhancing the bioremediation process (Kösesakal 2018). This symbiotic relationship between plants and microbes contributes to the observed reductions in hydrocarbon concentrations.
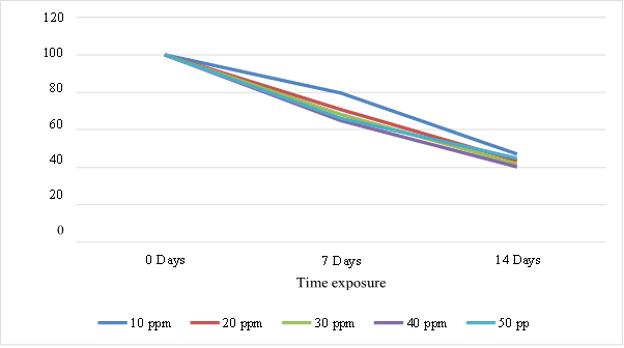
Fig. 3. Reduction of petroleum hydrocarbon concentrations (%) by A. microphylla in brackish water
The ability of A.microphylla to double its biomass is important in absorbing hydrocarbons in the test media. Miranda et al. (2020) stated that Azolla's ability to grow quickly, doubling its biomass in 4 days is the most important attribute in absorbing pollutants in nature. This fern can overcome hydrocarbon pollution through microbes that can potentially degrade hydrocarbons (Noyo et al. 2008).
Azolla species, such as Azolla pinnata and Azolla filiculoides, have shown promising potential in reducing petroleum hydrocarbons (PHs) in freshwater environments. Research indicates that A. pinnata can effectively phytoremediate freshwater contaminated with low levels of PHs, achieving a 92% degradation rate of total PHs within seven days (Mostafa et al. 2021, Parida et al. 2020). Similarly, A. filiculoides has demonstrated the ability to tolerate and degrade PHs, with biodegradation rates ranging from 71% to 94% for aliphatic and aromatic hydrocarbons at concentrations up to 0.2% in the growth medium (Kösesakal et al. 2016). Furthermore, A. filiculoides has shown efficacy in reducing 3-4 ring polycyclic aromatic hydrocarbons (PAHs) in the presence of crude oil, considering its potential for bioremediation in PAH-polluted freshwater areas (Kösesakal 2018). These findings highlight the valuable role of Azolla species in the remediation of petroleum-contaminated water bodies.
Azolla, a floating aquatic plant, significantly reduces petroleum hydrocarbons through its exceptional oil-absorbing capabilities and rapid growth rate. Azolla's unique hierarchical leaf surface structure enables it to absorb oil contaminants at the water/air interface efficiently, showcasing remarkable oil or organic solvent absorption capabilities. Additionally, Azolla's rapid growth, doubling in the area every 4-5 days, position it as a sustainable alternative to synthetic oil-cleaning materials, making it an effective natural solution for mitigating the negative impacts of oil spills and promoting a cleaner water ecosystem (Ghulam et al. 2024). Furthermore, Azolla can be used as a biofertilizer for dryland vegetable crops, reducing the need for synthetic nitrogen fertilizers derived from petroleum, thus indirectly contributing to reducing petroleum hydrocarbons in agricultural practices (Jama et al. 2023).
Azolla plays a crucial role in bioremediation processes by effectively mitigating the toxic effects of pollutants in various environmental settings. Studies have shown that Azolla species, such as Azolla pinnata and Azolla filiculoides, possess remarkable capabilities in chelating metal toxicants from water bodies (Rajalakshmi et al. 2923, Zazouli et al. 2023). These aquatic macrophytes exhibit strong antioxidant activity, high mineral content, and bioactivity against harmful bacteria, making them valuable biofiltering agents for wastewater treatment (Subpiramaniyam et al. 2023). Additionally, Azolla's ability to uptake, accumulate, and biodegrade pollutants like polycyclic aromatic hydrocarbons (PAHs) further highlights its potential for phytoremediation of contaminated water resources (Zazouli et al. 2023). Furthermore, Azolla's role extends beyond bioremediation, as it is also utilized in composting processes to enhance the quality of organic fertilizers, showcasing its versatility in sustainable agricultural practices (Ebrahim et al. 2024, Korsa et al. 2024).
3.2. Chlorophyll of A.microphylla
Chlorophyll is the main requirement for photosynthesis and an indicator of aquatic productivity. The presence of petroleum hydrocarbon pollutants in the test media can reduce the chlorophyll of A. microphylla leaves. This research shows that the higher the content of petroleum hydrocarbon pollutants, the lower the chlorophyll content of the fern leaves. The lowest chlorophyll levels were recorded in the treatment with a petroleum hydrocarbon concentration of 50 ppm, namely 5.300 mMole/m2 (Table 3).
Table 3. Chlorophyll content (mMole/m2) of A. microphylla leaves in brackish water contaminated PH
|
No. Treatments |
0 day |
7 day |
14 day |
|
1. A11 (PH at 10 ppm with AM) |
7.610 |
7.320 |
7.110 |
|
7.650 |
7.350 |
7.130 |
|
|
7.600 |
7.300 |
7.070 |
|
|
Average |
7.620 |
7.323 |
7.103 |
|
Standard of deviation |
0.026 |
0.025 |
0.031 |
|
2. A21 (PH at 20 ppm with AM) |
7.580 |
7.190 |
6.970 |
|
7.600 |
7.200 |
7.000 |
|
|
7.620 |
7.210 |
7.030 |
|
|
Average |
7.600 |
7.200 |
7.000 |
|
Standard of deviation |
0.020 |
0.010 |
0.030 |
|
3. A31 (PH at 30 ppm with AM) |
7.570 |
6.790 |
6.590 |
|
7.600 |
6.800 |
6.600 |
|
|
7.630 |
6.810 |
6.610 |
|
|
Average |
7.600 |
6.800 |
6.600 |
|
Standard of deviation |
0.030 |
0.010 |
0.010 |
|
4. A41 (PH at 40 ppm with AM) |
7.600 |
6.410 |
6.200 |
|
7.580 |
6.350 |
6.170 |
|
|
7.620 |
6.440 |
6.230 |
|
|
Average |
7.600 |
6.400 |
6.200 |
|
Standard of deviation |
0.020 |
0.046 |
0.030 |
|
5. A51 (PH at 50 ppm with AM) |
7.570 |
5.680 |
5.270 |
|
7.600 |
5.700 |
5.300 |
|
|
7.630 |
5.720 |
5.330 |
|
|
Average |
7.600 |
5.700 |
5.300 |
|
Standard of deviation |
0.030 |
0.020 |
0.030 |
|
6. A60 (PH at 0 ppm with AM) |
7.684 |
7.588 |
7.652 |
|
7.652 |
7.731 |
7.721 |
|
|
7.588 |
7.577 |
7.683 |
|
|
Average |
7.641 |
7.582 |
7.687 |
|
Standard of deviation |
0.049 |
0.008 |
0.049 |
Chlorophyll is an important plant stress indicator and is associated with chloroplast membrane peroxidation (Pandey & Gupta 2015). The decrease in A. microphylla chlorophyll concentration after exposure to high concentrations of petroleum hydrocarbons in A4 (40 ppm) and A5 (50 ppm) was due to the high concentration of petroleum hydrocarbons absorbed by the plants. The response of A. microphylla to increased absorption directly affects the decrease in plant leaf chlorophyll. According to Zakari et al. (2020), generally, a decrease in chlorophyll content occurs due to an increase in the levels of pollutants absorbed, resulting in oxidative damage to leaf tissue due to disruption of the function of carotenoids in the leaves, which affects the chlorophyll content of the leaves resulting in changes in leaf color (Shen et al. 2018).
Further effects when the concentration of petroleum pollutants is high can change the structure of chlorophyll, namely damage to the function and structure of chloroplasts so that metabolic processes cannot take place optimally. Chloroplasts are the organs most sensitive to pollutants (Kandasamy et al. 2021). The decrease in the chlorophyll content of A. microphylla leaves is caused by the increasing amount of pollutant material absorbed so that the absorption of other elements (Mg and Fe) that plants need for chlorophyll formation is reduced (Song et al. 2021).
Differences in petroleum hydrocarbon concentrations influenced chlorophyll levels in Azolla (p < 0.05). The absorption of petroleum hydrocarbons caused a decrease in the plants' chlorophyll (Figures 3 and 4). In Figure 4, the presence of hydrocarbon petroleum pollutants causes a sharp decrease in chlorophyll on the 7th and 14th days. The higher the pollutant concentration, the higher the decrease in the chlorophyll content of A. microphylla leaves (R2 = 0.8791 and 0.8052). The maximum decrease was on the 7th to the 14th day, ranging from 15.8 to 18.4%. The decrease was due to the fern's absorption of petroleum hydrocarbon pollutants. The higher the absorption of petroleum hydrocarbon pollutants, the higher the decrease in leaf chlorophyll.
Oil pollutants have been shown to impact chlorophyll content in vegetation significantly. Overall, these findings highlight the detrimental effects of oil pollutants on chlorophyll levels in plants, emphasizing the importance of monitoring and mitigating oil pollution to protect vegetation health and ecosystem stability. Studies have demonstrated that oil pollution alters the pigment content in plants, leading to changes in chlorophyll levels. These findings align with Onyia et al. (2020) and Otitoju & Onwurah (2010), who observed similar decreases in chlorophyll in plants exposed to hydrocarbon pollutants, highlighting chlorophyll degradation as a common response to oil-induced stress (Baruah et al. 2014). The decrease in chlorophyll content is often associated with stressed vegetation, as reflected in increased reflectance at chlorophyll absorption features and decreased reflectance at carotenoids and anthocyanins absorption features in polluted areas (Onyia et al. 2018).
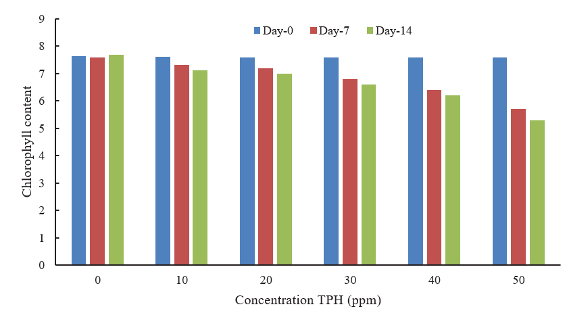
Fig. 4. Effect of petroleum hydrocarbons on chlorophyll of A. microphylla
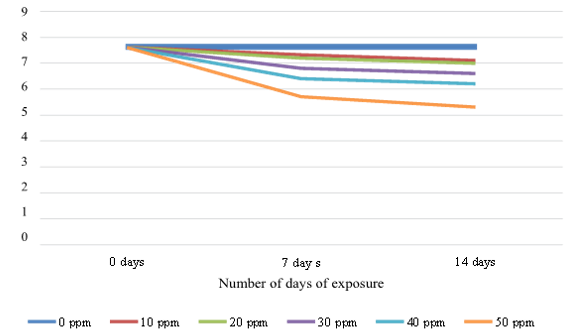
Fig. 5. Effect of PH on the reduction of chlorophyll of A. microphylla during the study
Oil pollutants have a significant impact on marine chlorophyll levels. Studies have shown that oil spills can lead to different stages of impact on aquatic environments, including the initial stage, where other pollutants' toxicity surpasses algae tolerance, a self-repairing stage where petroleum hydrocarbons become a carbon source for algal growth, and a long-term toxic affecting stage (Wang 2015). Additionally, research on the physiological responses of algae to petroleum pollution revealed that Nannochloropsis oculata exhibited more tolerance compared to Porphyridium cruentum, with varying growth responses depending on the concentration of water-soluble fractions of petroleum fuels (Ezenweani & Kadiri 2023). These findings highlight the complex interactions between oil pollutants and marine chlorophyll levels, emphasizing the need for monitoring and remediation strategies to mitigate the ecological impacts of oil contamination on marine ecosystems. While chlorophyll reduction may affect A. mycrophylla's long-term viability in highly polluted waters, its resilience at lower concentrations suggests the potential for sustained phytoremediation in moderately contaminated environments.
3.3. Water quality parameters
Water quality parameters were monitored to ensure stable conditions suitable for A. microphylla growth, with measurements remaining within normal ranges throughout the experiment. The results of measuring water quality parameters show that the water conditions are normal. Temperature ranges from 29.9-27.0°C, pH 6.5-7.1, salinity around 3, and dissolved oxygen ranges from 3.2-5.0 (Table 4).
Table 4. Results of measuring the range of water quality parameters during the research
|
Parameter |
Unit |
Results |
Quality Standards |
References |
|
Salinity |
ppt |
2.9-3.0 |
0-5 |
2, 4, & 5 |
|
Temperature |
oC |
27.00-29.9 |
28-30 |
2, 5 |
|
pH |
- |
6.5-7.1 |
7.5-8.5 |
1, 3, 5 |
|
Dissolved oxygen |
mg L-1 |
3.2-5.0 |
>3.0 |
5 |
1: APHA (2008); 2: Effendi et al (2020); 3: ESFA (2012); 4: Parbo et al. (2019); 5: RMMAF (2012).
4. Discussion
A. microphylla significantly affects the reduction of petroleum hydrocarbons in brackish water. Adding this fern can reduce levels of this pollutant by up to 36.6%. These results support using A. microphylla as an environmentally friendly phytoremediation agent in brackish water ecosystems affected by petroleum pollution. The observed time-dependent nature of the reduction also has a very real effect, where petroleum hydrocarbon levels decreased with time, including in the experimental control unit. However, in experimental control (without A. microphylla), the percentage reduction of petroleum hydrocarbon pollutants was lower, namely only 14-22%. The optimal reduction in petroleum hydrocarbons occurred in the experimental unit concentration of 30 ppt on the 14th day. This pollutant causes stress on A. microphylla, which can be seen from decreased leaf chlorophyll levels. However, this plant can be relied upon as an alternative to reduce levels of petroleum hydrocarbon pollutants, especially in brackish water, in the future.
The authors thank the Research and Community Service Institute, University of Riau, Pekanbaru, for financing the study under contract No. 168/UN19.5.1.3/PT.01.03/2022. We also thank the Marine Sciences Department and Aquaculture Department, University of Riau, Pekanbaru for their valuable support.
References
[APHA] American Public Health Association. (2008). Standard methods for the examination of water and wastewater. Washington D.C.
[ESFA] European Food Safety Authority. (2012). Scientific opinion on the risk for public health related to the presence of mercury and methylmercury in food. EFSA 10(12), 2985.
[RMMAF] Regulation of the Minister of Marine Affairs and Fisheries of the Republic of Indonesia. No. 75/PERMEN-KP/2016. (2016). General guidelines for growing tiger shrimp (Penaeus monodon) and vaname shrimp (Litopenaeus vannamei). Ministry of Marine Affairs and Fisheries of the Republic of Indonesia, 43 p.
Baruah, P., Saikia, R. R., Baruah, P. P., & Deka, S. (2014). Effect of crude oil contamination on the chlorophyll content and morpho-anatomy of Cyperus brevifolius (Rottb.) Hassk. Environmental Science and Pollution Research, 21(21), 12530-12538. https://doi.org/10.1007/S11356-014-3195-Y
Caronni, S., Quaglini, L. A., Franzetti, A., Gentili, R., Montagnani, C., & Citterio, S. (2023). Does Caulerpa prolifera with its bacterial coating represent a promising association for seawater phytoremediation of diesel hydrocarbons? Plants, 12(13). https://doi.org/10.3390/plants12132507
Dehnavi, S. M., & Ebrahimipour, G. (2022). Comparative remediation rate of biostimulation, bioaugmentation, and phytoremediation in hydrocarbon contaminants. International Journal of Environmental Science and Technology, 19(11), 11561-11586. https://doi.org/10.1007/s13762-022-04343-0
Ebrahim, N., Mahmoud, S., EL-Shahat, R., & Mohamed, H. (2024). Azolla as a source of organic nitrogen and its role in recycling agricultural residues and improving the quality of compost. Assiut Journal of Agricultural Sciences, 55(2), 246-259. https://doi.org/10.21608/ajas.2024.259605.1321
Effendi, I., Saputra, E., Tanjung, A., & Elizal, E. (2020). Adaptation of Azolla mycrophyla to brackish water ecosystem. IOP Conference Series: Earth and Environmental Science, 469. https://doi.org/10.1088/1755-1315/469/1/012024
Ezenweani, R. S., & Kadiri, M. S. (2023). Resilience and bioresponse of two marine algae to petroleum fuel pollution. Applied phycology, 4(1), 54-77. https://doi.org/10.1080/26388081.2023.2185815
Galal, T. M., Eid, E. M., Dakhil, M. A., & Hassan, L. M. (2018). Bioaccumulation and rhizofiltration potential of Pistia stratiotes L. for mitigating water pollution in the Egyptian wetlands. International Journal of Phytoremediation, 20(5), 440-44. https://doi.org/10.1080/15226514.2017.1365343
Ghulam, M., Bhat, I. M., Kakroo, I., Balachandran, A., Tabasum, R., Majid, K., Wani, F.M., Manna, U., Ghodake, G., & Lone, S. (2024). Azolla pinnata: Sustainable floating oil cleaner of water bodies. ACS omega. https://doi.org/10.1021/acsomega.3c08417
Golzary, A., Abdoli, M. A., Yoshikawa, K., Khodadadi, A., & Karbassi, A. (2019). Azolla as a feedstock for bio- refinery: Cultivation, conversion and application. https://doi.org/10.5339/qfarc.2016.eesp2082
Hamdan, H .Z., & Houri, A. F. (2022). CO2 sequestration by propagation of the fast-growing Azolla spp. Environmental Science and Pollution Research, 29(12). https://doi.org/10.1007/s11356-021-16986-6
Hardestyariki, D., & Fitria, S. (2023). Potential of Neptunia oleracea L. as a phytoremediation agent for petroleum liquid waste. Journal of Ecological Engineering, 24(5). https://doi.org/10.12911/22998993/161296
Jama, A., Widiastuti, D. P., Gafur, D., & Davis, J. G. (2023). Azolla biofertilizer is an effective replacement for urea fertilizer in vegetable crops. Sustainability, 15(7), 6045-6045. https://doi.org/10.3390/su15076045
Kandasamy, S., Narayanan. M., He, Z., Liu, G., Ramakrishnan, M., Thangavel, P., Pugazhendhi, A., Raja, R., & Carvalho, I.S. (2021). Current strategies and prospects in algae for remediation and biofuels: An overview. Biocatalysis and Agricultural Biotechnology, 35, 102045. https://doi.org/10.1016/j.bcab.2021.102045
Korsa, G., Alemu, D., & Ayele, A. (2024). Azolla plant production and their potential applications. International Journal of Agronomy, 2024(1). https://doi.org/10.1155/2024/1716440
Kösesakal, T. (2018). Assessment of the biodegradation capacity of Azolla on polycyclic aromatic hydrocarbons in crude oil. Global Nest Journal, 20(3). https://doi.org/10.30955/gnj.002544
Kösesakal, T., Ünal, M., Kulen, O., Memon, A., & Yüksel, B. (2016). Phytoremediation of petroleum hydrocarbons by using a freshwater fern species Azolla filiculoides Lam. International Journal of Phytoremediation, 18(5). https://doi.org/10.1080/15226514.2015.1115958
Kumari, A., Kaur, R., & Kaur, R. (2019). A review on fate and remediation techniques of oil spills. International Journal of Research in Pharmaceutical Sciences, 10(1). https://doi.org/10.26452/ijrps.v10i1.1786
Miranda, A.F., Biswas, B., Ramkumar, N., Singh, R., Kumar, J., James, A., Roddick, F., Lal, B., Subudhi, S., Bhaskar, T., & Mouradov, A. (2016). Aquatic plant Azolla as the universal feedstock for biofuel production. Biotechnology for Biofuels, 9(1). https://doi.org/10.1186/s13068-016-0628-5
Miranda, A. F., Kumar, N. R., Spangenberg, G., Subudhi, S., Lal, B., & Mouradov, A. (2020). Aquatic plants, landoltia punctata, and azolla filiculoides as bio‐converters of wastewater to biofuel. Plants, 9(4). https://doi.org/10.3390/plants9040437
Mostafa, A. A., Hegazy, A. K., Mohamed, N. H., Hafez, R. M., Azab, E., Gobouri, A. A., Saad, H. A., Abd-El Fattah, A. M., & Mustafa, Y. M. (2021). Potentiality of azolla pinnata r. Br. for phytoremediation of polluted freshwater with crude petroleum oil. Separations, 8(4), 39. https://doi.org/10.3390/separations8040039
Nedi, S., Effendi, I., Tanjung, A., & Elizal, E. (2023). Reduction of hydrocarbon pollutants by hyacinth plants (Eichhornia crassipes). F1000Research, 12. https://doi.org/10.12688/f1000research.131846.1
Nedi, S., Pramudya, B., Riani, E., & Manuwoto, M. (2010). Karakteristik lingkungan perairan Selat Rupat. Journal of Environmental Science, 4(1), 25-35.
Noyo, E. E., Okoloko, E. G., & Agbogidi, O. M. (2008). Physical and ionic characteristics in water soluble fraction (WSF) of Olomoro well-head crude oil before and after exposure to Azolla africana Desv. African Journal of Biotechnology, 7, 35-40.
Onyia, N. N., Balzter, H., & Berrio, J. C. (2018). Detecting vegetation response to oil pollution using hyperspectral
indices. IGARSS 2018 – 2018 IEEE International Geoscience and Remote Sensing Symposium, Valencia, Spain,
3963-3966. https://doi.org/10.1109/IGARSS.2018.8519398
Onyia, N. N., Balzter, H., & Berrio, J. C. (2020). Evaluating the performance of vegetation indices for detecting oil pollution effects on vegetation using hyperspectral (Hyperion EO-1) and multispectral (Sentinel-2A) data in the Niger Delta. Hyperspectral Remote Sensing: Theory and Applications, 377-399.
https://doi.org/10.1016/B978-0-08-102894-0.00018-8
Otitoju., Onwurah. (2010). Chlorophyll contents of oil palm (Elaeis Guineensis) leaves harvested from crude oil polluted soil: a shift in productivity dynamic. Annals of Biological Research, 1(4), 1-8.
Pandey, C., & Gupta, M. (2015). Selenium and auxin mitigates arsenic stress in rice (Oryza sativa L.) by combining the role of stress indicators, modulators and genotoxicity assay. Journal of Hazardous Materials, 287, 384-391. https://doi.org/10.1016/j.jhazmat.2015.01.044
Parbo, A. P., Effendi, I., & Nedi, S. (2019). The effect of detergents to the growth of Azolla microphylla in brackish water. Asian Journal of Aquatic Sciences, 2(2), 145-152.
Parida, P., Satapathy, K. B., & Mohapatra, A. (2020). Phytoremediation potential of aquatic macrophyte azolla pinnata R.BR. And Salvinia molesta mitchell to remove chromium from waste water. Plant Archives, 20(1), 2595-2601.
Rajalakshmi, K. S. V., & Paari K. (2023). A comprehensive study on the assessment of chemically modified Azolla pinnata as a potential cadmium sequestering agent. International Journal of Experimental Research and Review. https://doi.org/10.52756/ijerr.2023.v36.001
Sekhar, V., Umakrishna, K., Srinivasa Rao, V., & Satyavani, C. (2019). Completely randomised design (CRD) analysis
– by manual and MS-Excel. International Journal of Current Microbiology and Applied Sciences, 8(03), 954-959. https://doi.org/10.20546/ijcmas.2019.803.114
Shen, Y., Li, J., Gu, R., Yue, L., Wang, H., Zhan, X., & Xing, B. (2018). Carotenoid and superoxide dismutase are the most effective antioxidants participating in ROS scavenging in phenanthrene accumulated wheat leaf. Chemosphere, 197, 513-525. https://doi.org/10.1016/j.chemosphere.2018.01.036
Song, K., Gao, J., Li, S., Sun, Y., Sun, H., An, B., … He, X. (2021). Experimental and theoretical study of the effects of rare earth elements on growth and chlorophyll of alfalfa (Medicago sativa L.) Seedling. Frontiers in Plant Science, 12. https://doi.org/10.3389/fpls.2021.731838
Subpiramaniyam, S., Hong, S. C., Yi, P. I., Jang, S. H., Suh, J. M., Jung, E. S., & Kim, J. S. (2023). Biochemical responses and phytoremediation potential of Azolla imbricata (Roxb.) Nakai in water and nutrient media exposed to waste metal cutting fluid along with temperature and humidity stress. Journal of Hazardous Materials, 451. https://doi.org/10.1016/j.jhazmat.2023.131101
Wang, D. (2015). Remote sensing-based study on the temporal variations in chlorophyll-a concentration after confo oil spill in Bohai Sea. Journal of The Indian Society of Remote Sensing, 43(1), 133-142.
https://doi.org/10.1007/S12524-014-0381-9
Wang, L., Ji, B., Hu, Y., Liu, R., & Sun, W. (2017). A review on in situ phytoremediation of mine tailings. Chemosphere, 184. https://doi.org/10.1016/j.chemosphere.2017.06.025
Zakari, S. A., Asad, M. A. U., Han, Z., Zhao, Q., & Cheng, F. (2020). Relationship of Nitrogen deficiency-induced leaf senescence with ROS generation and ABA concentration in rice flag leaves. Journal of Plant Growth Regulation, 39, 1503-1517. https://doi.org/10.1007/s00344-020-10128-x
Zazouli, M. A., Ala, A., Asghari, S., & Babanezhad, E. (2023). Evaluation of Azolla filiculoides potential in pyrene and phenanthrene accumulation and phytoremediation in contaminated waters. International Journal of Phytoremediation, 1-10. https://doi.org/10.1080/15226514.2023.2257314
AMA Style
Nedi S, Effendi I, Tanjung A, Kurniawan R, Elizal E. Phytoremediation of Brackish Waters Polluted by Petroleum Hydrocarbon by Using Azolla microphylla. Rocznik Ochrona Środowiska. 2025; 27. https://doi.org/10.54740/ros.2025.001
ACM Style
Nedi, S., Effendi, I., Tanjung, A., Kurniawan, R., Elizal, E. 2025. Phytoremediation of Brackish Waters Polluted by Petroleum Hydrocarbon by Using Azolla microphylla. Rocznik Ochrona Środowiska. 27. DOI:https://doi.org/10.54740/ros.2025.001
ACS Style
Nedi, S.; Effendi, I.; Tanjung, A.; Kurniawan, R.; Elizal, E. Phytoremediation of Brackish Waters Polluted by Petroleum Hydrocarbon by Using Azolla microphylla Rocznik Ochrona Środowiska 2025, 27, 1-12. https://doi.org/10.54740/ros.2025.001
APA Style
Nedi, S., Effendi, I., Tanjung, A., Kurniawan, R., Elizal, E. (2025). Phytoremediation of Brackish Waters Polluted by Petroleum Hydrocarbon by Using Azolla microphylla. Rocznik Ochrona Środowiska, 27, 1-12. https://doi.org/10.54740/ros.2025.001
ABNT Style
NEDI, S.; EFFENDI, I.; TANJUNG, A.; KURNIAWAN, R.; ELIZAL, E. Phytoremediation of Brackish Waters Polluted by Petroleum Hydrocarbon by Using Azolla microphylla. Rocznik Ochrona Środowiska, v. 27, p. 1-12, 2025. https://doi.org/10.54740/ros.2025.001
Chicago Style
Nedi, Syahril, Effendi, Irwan, Tanjung, Afrizal, Kurniawan, Ronal, Elizal, Elizal. 2025. "Phytoremediation of Brackish Waters Polluted by Petroleum Hydrocarbon by Using Azolla microphylla". Rocznik Ochrona Środowiska 27, 1-12. https://doi.org/10.54740/ros.2025.001
Harvard Style
Nedi, S., Effendi, I., Tanjung, A., Kurniawan, R., Elizal, E. (2025) "Phytoremediation of Brackish Waters Polluted by Petroleum Hydrocarbon by Using Azolla microphylla", Rocznik Ochrona Środowiska, 27, pp. 1-12. doi:https://doi.org/10.54740/ros.2025.001
IEEE Style
S. Nedi, I. Effendi, A. Tanjung, R. Kurniawan, E. Elizal, "Phytoremediation of Brackish Waters Polluted by Petroleum Hydrocarbon by Using Azolla microphylla", RoczOchrSrod, vol 27, pp. 1-12. https://doi.org/10.54740/ros.2025.001
| Received: August 2024 | Accepted: December 2024 | Published: January 2025 |

